Thermite
_small.jpg) A thermite reaction (sometimes called a "Goldschmidt reaction") refers to a very exothermic process occurring between a metal Oxide and a more active pure metal. The more reactive metal reduces the metal Oxide, Oxidizing itself and releasing a substantial amount of energy during the reaction.
A thermite reaction (sometimes called a "Goldschmidt reaction") refers to a very exothermic process occurring between a metal Oxide and a more active pure metal. The more reactive metal reduces the metal Oxide, Oxidizing itself and releasing a substantial amount of energy during the reaction.
Generally, thermite is made by mixing Iron Oxide and Aluminum powder and igniting it at very high temperatures (a few thousand degrees). The reaction releases so much energy, molten Iron metal is produced as one of the products.
The two most common types of thermite are made using either Iron(III) Oxide, Fe2O3 (also known as Hematite), or using Iron(II, III) Oxide, Fe3O4 (also known as Magnetite). The Iron Oxide is mixed with finely powdered Aluminum metal. When the thermite reacts, liquid Iron metal and Aluminum Oxide, Al2O3, is produced as a result.
-small.jpg) Other, more exotic forms of thermite can also be produced. Using other metal Oxides, one can produce other, sometimes more powerful, blends of thermite. For instance, substituting Copper(II) Oxide for Iron Oxide in a thermite mixture can produce a very brightly burning reaction which yields Copper metal as a result. Although Copper Oxide thermite is probably the most common of the exotic thermites, one could also use other metal Oxides such as Tin Oxide, Lead Oxide, or any other metal Oxide which could be reacted with a reducing metal (such as Aluminum or Magnesium). They key is that the reducing metal must be sufficiently higher on the activity series than the metal Oxide in order to support the single replacement reaction.
Other, more exotic forms of thermite can also be produced. Using other metal Oxides, one can produce other, sometimes more powerful, blends of thermite. For instance, substituting Copper(II) Oxide for Iron Oxide in a thermite mixture can produce a very brightly burning reaction which yields Copper metal as a result. Although Copper Oxide thermite is probably the most common of the exotic thermites, one could also use other metal Oxides such as Tin Oxide, Lead Oxide, or any other metal Oxide which could be reacted with a reducing metal (such as Aluminum or Magnesium). They key is that the reducing metal must be sufficiently higher on the activity series than the metal Oxide in order to support the single replacement reaction.
Thermite has found some use as a crude method of welding metals due to the intense heat and molten metals produced by the reaction.
Thermite reactions can also be used on occasion to produce pure metals from their oxide counterparts as long as the reaction taking place is thermodynamically favorable
(decrease in Gibbs Free Energy).
Thermite is not easy to ignite. Thermite has a very high activation energy required to start the reaction. The two most common ways to ignite thermite are:
- Magnesium Ribbon (Mg)
- Magnesium metal burns in an Oxygen environment (air) in a very bright, exothermic reaction. Magnesium ribbon can burn at several thousand degrees easily igniting thermite. The Magnesium ribbon is useful as it acts like a fuse, calmly burning, allowing a short delay between when the ribbon is lit and when the thermite begins to react.
Other forms of Magnesium metal can be substituted for Magnesium ribbon such as metal turnings, powders, or even common sparkers which contain Magnesium.
- Potassium Permanganate (KMnO4) + Glycerin
- An alternative to using Magnesium ribbon is to use the heat given off by the reaction between Potassium Permanganate and glycerin. Potassium Permanganate is an extremely powerful Oxidizer which spontaneously ignites after coming in contact with glycerin.
After adding a few drops of glycerin to Potassium Permanganate powder and a short delay, a violent exothermic oxidation reaction occurs which will ignite a thermite mixture.
It is important to mix the thermite ingredients thoroughly in order to create a homogeneous mixture. Unless the thermite is sufficiently mixed, it may be difficult to ignite or sustain the thermite reaction.
Thermite Types (by metal Oxide):
- Iron(III) Oxide - Fe2O3
- Iron(II, III) Oxide - Fe3O4
- Copper(II) Oxide - CuO
- Copper(I) Oxide - Cu2O
- Tin(IV) Oxide - SnO2
- Titanium(IV) Oxide - TiO2
- Manganese(IV) Oxide - MnO2
- Manganese(III) Oxide - Mn2O3
- Chromium(III) Oxide - Cr2O3
- Cobalt(II) Oxide - CoO
- Silicon Dioxide - SiO2
- Nickel(II) Oxide - NiO
- Vanadium(V) Oxide - V2O5
- Silver(I) Oxide - Ag2O
- Molybdenum(VI) Oxide - MoO3
Click Here To See
>>> ** Videos / Pictures of Thermite Demonstrations ** <<<
Iron(III) Oxide [Fe2O3]
.jpg)

Iron(III) Oxide is a reddish-brown powder (left), commonly known as "rust" is mixed with Aluminum Powder (center) to make thermite (right).
The balanced chemical reaction between Iron(III) Oxide and Aluminum is show below,
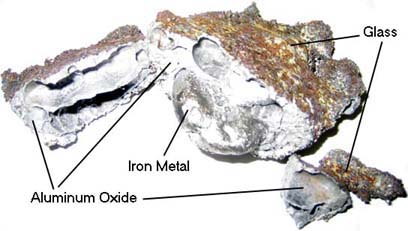
Above: Lumps of Iron metal produced by an Iron(III) Oxide thermite reaction.
According to the reaction's stoichiometry, the ratio of Fe2O3 to Aluminum powder by weight is about 3 to 1 (2.96 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -768.75 kJ assuming that both the Iron metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -851.50 kJ per 213.65 grams of thermite (-3.985 kJ/g).
Iron(II, III) Oxide [Fe3O4]
.jpg)

Iron(II, III) Oxide is a black powder (above), sometimes known as "Magnetite" due to its magnetic properties.
According to the reaction's stoichiometry, the ratio of Fe3O4 to Aluminum powder by weight is about 3.2 to 1 (3.22 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -3002.79 kJ assuming that both the Iron metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -3347.60 kJ per 910.46 grams of thermite (-3.677 kJ/g).
Copper(II) Oxide [CuO]


Copper(II) Oxide, or "Cupric Oxide", is a black powder shown above (left) as is CuO Thermite (right).
According to the reaction's stoichiometry, the ratio of CuO to Aluminum powder by weight is about 4.4 to 1 (4.42 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -1108.89 kJ assuming that both the Copper metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -1203.8 kJ per 262.60 grams of thermite (-4.584 kJ/g).
Copper(I) Oxide [Cu2O]

Copper(I) Oxide, or "Cuprous Oxide", is a reddish colored powder which, when mixed with Aluminum powder, forms the thermite shown above.
According to the reaction's stoichiometry, the ratio of Cu2O to Aluminum powder by weight is about 8.0 to 1 (7.96 to 1 to be more exact).
The change in entropy of this reaction is calculated to be, ΔH = -1035.21 kJ assuming that both the Copper metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -1169.8 kJ per 483.23 grams of thermite (-2.421 kJ/g).
Tin(IV) Oxide [SnO2]
_Oxide_small.jpg)

Tin(IV) Oxide, or "Stannic Oxide", is a white powder is above (left) as is Tin(IV) Oxide thermite (right).
Above: Tin metal which was extracted from the remains of a Tin(IV) Oxide thermite reaction and recast into shiny round lumps..
According to the reaction's stoichiometry, the ratio of SnO2 to Aluminum powder by weight is about 4.2 to 1 (4.19 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -1477.95 kJ assuming that both the Tin metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -1609.30 kJ per 560.05 grams of thermite (-2.873 kJ/g).
Titanium(IV) Oxide [TiO2]
_Oxide_small.jpg)
Titanium(IV) Oxide, or "titania", is a white powder (above).
Above: Lumps of Titanium metal produced from a KClO3 boosted TiO2 thermite reaction.
According to the reaction's stoichiometry, the ratio of TiO2 to Aluminum powder by weight is about 2.2 to 1 (2.22 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -366.69 kJ assuming that both the Titanium metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -519.40 kJ per 347.52 grams of thermite (-1.495 kJ/g).
In practice, however, the reaction does not appear to proceed as described above. The Aluminum metal does not seem to reduce the Titanium(IV) Oxide all the way down to Titanium metal but rather stops at a less-oxidized state of Titanium. A black Titanium Oxide, which is likely to be Titanium(III, IV) Oxide, is left after the reaction ceases. Upon analysis, one can further reduce the black Titanium Oxide further using Magnesium as a reducing agent. Doing so one can obtain a golden-yellow colored substance which is presumably Titanium(II) Oxide.
Titanium(II) Oxide, TiO, is said to be golden-yellow colored,
Titanium(III) Oxide, Ti2O3, is said to be violet colored, and
Titanium(III, IV) Oxide, Ti3O5, is said to be black colored.It has been shown that one can use Potassium Chlorate (KClO3) to boost TiO2 thermite reactions. With the addition of Potassium Chlorate, extra Aluminum powder, and a fluxing agent (Fluorspar, CaF2) to the thermite mixture, elemental Titanium can be produced.
Mixing the ingredients TiO2, Al, KClO3, and CaF2 using the ratio of 100 : 72 : 61 : 47 respectively by weight*, one can achieve a fast-burning thermite reaction which produces Titanium metal.
(* Ideal ratio still in development)
Manganese(IV) Oxide [MnO2]
_Oxide_small.jpg)
Manganese(IV) Oxide is a black powder (above).
Above: Nuggets of Manganese metal produced by a MnO2 thermite reaction
According to the reaction's stoichiometry, the ratio of MnO2 to Aluminum powder by weight is about 2.4 to 1 (2.42 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -1639.71 kJ assuming that both the Manganese metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -1788.7 kJ per 368.74 grams of thermite (-4.851 kJ/g).
Manganese(III) Oxide [Mn2O3]

_Oxide_thermite_small.jpg)
Manganese(III) Oxide is a dark brownish-black powder (left); Mn2O3 thermite (right).
According to the reaction's stoichiometry, the ratio of Mn2O3 to Aluminum powder by weight is about 2.9 to 1 (2.93 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -648.7 kJ assuming that both the Manganese metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -716.7 kJ per 211.83 grams of thermite (-3.38 kJ/g).
Chromium(III) Oxide [Cr2O3]
_Oxide_small.jpg)

Chromium(III) Oxide is a green powder shown above (left), as is Chromium(III) Oxide thermite (right).
According to the reaction's stoichiometry, the ratio of Cr2O3 to Aluminum powder by weight is about 2.8 to 1 (2.82 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -501.87 kJ assuming that both the Chromium metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -536.0 kJ per 205.95 grams of thermite (-2.603 kJ/g).
Cobalt(II) Oxide [CoO]

Cobalt(II) Oxide is a black powder shown above (left). Cobalt(II) Oxide thermite (right).
Above: Small pieces of Cobalt metal produced from a CoO thermite reaction
According to the reaction's stoichiometry, the ratio of CoO to Aluminum powder by weight is about 4.2 to 1 (4.17 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -858.69 kJ assuming that both the Cobalt metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -962.0 kJ per 278.75 grams of thermite (-3.451 kJ/g).
Silicon Dioxide [SiO2]



Silicon Dioxide, in the form of common sand, shown above (left), Sulfur (center), and SiO2 thermite (right)..
Above: Large lump of elemental Silicon produced by a SiO2 thermite reaction
According to the reaction's stoichiometry, the ratio of SiO2 to Aluminum powder by weight is about 1.7 to 1 (1.67 to 1 to be more exact). However, using a simple stoichiometric ratio of only Silicon Dioxide and Aluminum powder will make the mixture extremely difficult to ignite. In order ignite the thermite more easily one can add extra Aluminum powder and Sulfur to the thermite mixture. Aluminum powder and Sulfur will react together in an extremely exothermic reaction and will burn at a high enough temperature so as to ignite and maintain the SiO2 and Aluminum powder reaction.
A mixture of Silicon Dioxide, Aluminum powder, and Sulfur in the ratio of 9 : 10 : 12 by weight respectively, works well and is (relatively) easy to ignite.
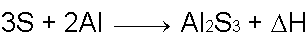
Silicon Dioxide and Aluminum powder react to form Aluminum Oxide and elemental Silicon. Another reaction, between Sulfur and Aluminum powder, aids the SiO2 thermite reaction and produces Aluminum Sulfide as a result.
Aluminum Sulfide will react with water, or moisture in the air, to give off the foul smelling and toxic Hydrogen Sulfide (H2S) gas, so avoid getting the products of the reaction wet.Alternatively, one can boost a SiO2 thermite reaction with the addition of Potassium Chlorate, and a fluxing agent (Fluorspar, CaF2) to the mixture and eliminate the need to use Sulfur as in the method described above.
Mixing SiO2, Al, KClO3, and CaF2 using the ratio of 100 : 96 : 81 : 55, one can create a fast-burning, and easy-to-ignite, SiO2 thermite reaction which has been shown to produce elemental Silicon as a product. The elimination of Sulfur from the thermite relieves one of the undesirable production of Aluminum Sulfide which, when wet, releases harmful and foul-smelling, H2S gas.
Nickel(II) Oxide [NiO]


Nickel(II) Oxide is a green powder shown above (left). Nickel(II) Oxide thermite (right).
Above: Lump of Nickel metal produced during a NiO thermite reaction
According to the reaction's stoichiometry, the ratio of NiO to Aluminum powder by weight is about 4.2 to 1 (4.15 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -883.3 kJ assuming that both the Nickel metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -955.4 kJ per 278.03 grams of thermite (-3.44 kJ/g).
Vanadium(V) Oxide [V2O5]


Vanadium(V) Oxide, also known as Vanadium Pentoxide, is a yellowish-orange powder shown above (left). Vanadium(V) Oxide thermite (right).
Above: Chunk of Vanadium metal from thermite reaction; colorful oxidization layer visible on surface
According to the reaction's stoichiometry, the ratio of V2O5 to Aluminum powder by weight is about 2.0 to 1 (2.02 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -3429.6 kJ assuming that both the Vanadium metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -3726.7 kJ per 815.44 grams of thermite (-4.57 kJ/g).
Silver(I) Oxide [Ag2O]


Silver(I) Oxide is a black powder shown above (left). Partially reacted Silver(I) Oxide thermite (right).
Above: A small nugget of Silver metal produced via a Silver(I) Oxide thermite reaction. Silver has been polished with a rotary grinder to reveal the shiny metal underneath the slag from the thermite reaction.
According to the reaction's stoichiometry, the ratio of Ag2O to Aluminum powder by weight is about 12.9 to 1 (12.88 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -1459.75 kJ assuming that both the Silver metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -1582.55 kJ per 749.18 grams of thermite (-2.11 kJ/g).
Molybdenum(VI) Oxide [MoO3]
According to the reaction's stoichiometry, the ratio of MoO3 to Aluminum powder by weight is about 2.67 to 1 (2.667 to 1 to be more exact).
The change in enthalpy of this reaction is calculated to be, ΔH = -875.4 kJ assuming that both the Molybdenum metal and Aluminum Oxide are in the liquid state after the reaction, as they solidify, they release additional energy, bringing the total change in enthalpy to, ΔH = -930.53 kJ per 197.9 grams of thermite (-4.70 kJ/g).
Last updated: 1/1/2012














