What are chemical reactions?
Chemical reactions are processes in which one or more reactants are converted into products through a chemical change involving the breaking and reforming of chemical bonds.
During a chemical reaction, the substances are converted into new substances which may exhibit totally different chemical properties than the original reactants. Mass is always conserved in purely chemical reactions, the mass of the reactants at the star of the reaction will always equal the mass of products produced at the end of the reaction. The total number of atoms involved in a reaction is also conserved meaning that even though atoms in molecules may be in completely new bonds after the reaction is complete, the total number of particles will be the same.
Chemical reactions must be balanced due to the fact that mass and the number of particles are conserved throughout the reaction. Take the following reaction for example; Hydrogen gas reacts with Oxygen gas to form water,
H2 + O2 → H2O
Notice that there are two Hydrogen atoms and two Oxygen atoms on the left hand side (the reactants) as denoted by the subscript 2 next to each symbol. On the right hand side (the products) there are two Hydrogen atoms but only one Oxygen atom. This reaction cannot be written correctly as shown since the number of atoms is not conserved on each side. Also, if one finds the mass of the products (18.02 grams), it does not equal the mass of the reactants (32.02 grams).
Through the use of coefficients added in front of each compound in the reaction, one is able to balance out the masses of each of the elements involves in the reaction. One should always use the simplest whole-number coefficients to balance chemical reactions.
The balanced reaction between Hydrogen and Oxygen gas is shown below,
![]()
The letter (g) shown in parenthesis after each substance in the chemical reaction indicates that each substance is in the gas phase. It is also possible for a chemical reaction to involve reactants or products in the solid (s) or liquid (l) phase, it is also common to see aqueous (aq) indicating the substance is insolution with water.
Tips for Balancing Chemical Reactions:
- Balance elements other than Hydrogen and Oxygen first
- Balance polyatomic ions as a group if they appear on both sides of the reaction
- Balance separately the elements that appear by themselves
- Use smallest whole-number coefficients
More detain on the process of balancing chemical reactions and Stoichiometry can be found in the external links section in the top left section of this page.
Stoichiometry is an absolutely critical skill to master in chemistry as so many concepts are based on understanding the relationships between the reactants and products in chemical reactions.
Types of Chemical Reactions
Synthesis (AKA combination)
Reactants combine to form products
Example, Sodium metal and Chlorine gas combine to form Sodium Chloride (Table Salt),
Decomposition
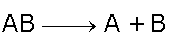
Reactants split up to form products
Example, Potassium Chlorate decomposes with heat to form Potassium Chloride and Oxygen gas.
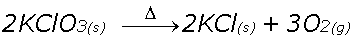
Single Replacement (AKA Single Displacement)
A more reactive substance will replace a less reactive substance in a compound
Example, Zinc metal displaces a Copper+2 ion in solution, precipitating out Copper metal.
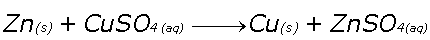
Double Replacement (AKA Double Displacement or Metathesis)
Reactants trade ions, anions (-) and cations (+) switch partners
Example, two colorless solutions of Lead Nitrate and Potassium Iodide react to form Potassium Nitrate and a bright yellow Lead Iodide precipitate.
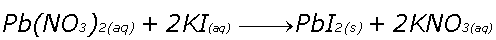
Combustion
A Hydrocarbon completely combusts with Oxygen gas to produce Carbon Dioxide and Water vapor.
Example, Methane (Natural gas) burns with excess Oxygen to form Carbon Dioxide and Water.
Indicators that a chemical reaction has occurred are:
- Color Change
- Precipitate
- Gas evolved
- A change in temperature
- Light
- Odor
Energy
Frequently in chemical reactions, there is an exchange of energy between the reaction and its surroundings. There are three possible situations which may occur involving the flow of energy in a chemical reaction, energy may be released (Exothermic), energy may be absorbed (Endothermic), or the rare instance when there is no over all change during the reaction.
Chemical bonds require an input of energy to be broken, however, when bonds are formed, energy is released. When a reaction is preformed at a constant pressure, the amount of energy released is given by the change in enthalpy of the system (ΔH). Although one cannot measure exact values for the total enthalpy of a system, it is possible to measure the change in enthalpy in a reaction and from this obtain a standard enthalpy of formation (ΔHfo). The Standard enthalpy of formation of a substance indicates the change in enthalpy which occurs under standard conditions (273.15 Kelvin, 1 atm) when that substance is formed. Using known values for ΔHfo, one can calculate the change in enthalpy in other reactions by finding the difference between the enthalpy of formation values of the products and the reactants.
By convention, the standard enthalpies of formation of any element in its natural state are always zero. For example, solid Iron metal is has an enthalpy of formation of zero, however, liquid Iron (although still a pure element) does not since Iron's natural state at 273.15 Kelvin [0 °C] is solid. Hydrogen gas (H2), for example, is a diatomic element, meaning that in its natural state it is found covalently bonded with another Hydrogen atom, a long Hydrogen atom (H) would not have a zero enthalpy of formation.
When the change in enthalpy of a reaction is negative (ΔH < 0), the reaction releases energy to the surroundings and is said to be exothermic. When the change in enthalpy of positive (ΔH > 0), the reaction absorbes energy from its surroundings and is said to be endothermic.
For example, In the reaction between Hydrogen gas and Oxygen gas to form Water,
2H2 (g) + O2 (g) → 2H2O (g)
Since both the Hydrogen gas and Oxygen gas on the reactant side of the equation have standard enthalpies of zero in the gas state, the total enthalpy of the reactants is 2(0) + 1(0) = 0 Joules. Water vapor (steam) on the other hand does have a non-zero enthalpy. In the gas state, water has an enthalpy of formation of -241820 Joules, meaning that the over all enthalpy of the products is 2(-241820) or -483640 Joules.
ΔH = HProducts - HReactants = -483640 J - 0 J = -483640 J
Since ΔH < 0, the reaction is exothermic and releases 4.8364 kJ of energy.
Last updated:
03/19/2006


