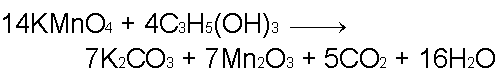Potassium Permanganate (KMnO4)
 Potassium Permanganate
(shown left) is a dark powder which, when dissolved in water,
disassociates into K+1 and MnO4-1
ions to form a deep purple solution.
Potassium Permanganate
(shown left) is a dark powder which, when dissolved in water,
disassociates into K+1 and MnO4-1
ions to form a deep purple solution.
The Permanganate ion (MnO4-1) acts as an
extremely powerful oxidizing agent in many chemical reactions.
Potassium Permanganate is such a powerful oxidizer, in fact,
that when mixed with certain substances, a combustion reaction
will proceed spontaneously without the need for a form of
ignition.
When
glycerin is poured onto a pile Potassium Permanganate powder,
the Potassium Permanganate quickly begins to react,
automatically starting a combustion reaction within seconds as
shown in the video below.
Due to the intense heat liberated in the process, as well as
the ease of starting the reaction, Potassium Permanganate /
Glycerin is sometimes used to ignite thermite mixtures.
|
Video
|
|---|
Last updated:
08/16/2006