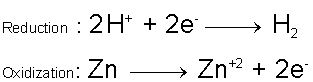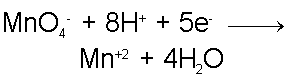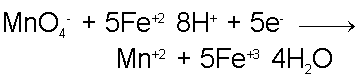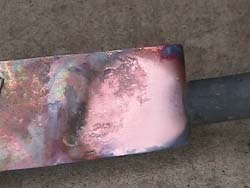Redox Reactions
Redox Reactions
A “redox” reaction involves the reduction and oxidation of the reactants, thereby changing the oxidation numbers of atoms taking part in the chemical reaction, through an exchange of electrons.
Examples of well-known redox reactions include the rusting of metal, the chemical reaction inside a battery, and combustion of hydrocarbons.
The
roaring fire shown to the left is an example of the rapid
oxidization
of the hydrocarbons making up the wood and the reduction of
the Oxygen
gas from the air. The, very rusty, Iron hammer to the bottom
right is
also being oxidized by the Oxygen in the air, but at a much
slower rate
than the burning wood.
Oxidation Number
Rules for determining oxidation numbers:
- The oxidation number of a neutral element is zero.
- Fluorine always has an oxidation number of -1 in compounds.
- The elements of groups IA (e.g., Na, K), IIA (e.g., Mg, Ca), and IIIA (e.g., Al, Ga) always have positive oxidation numbers of +1, +2, and +3, respectively, in compounds.
- Hydrogen has an oxidation number of +1 in all its compounds except in binary and ternary compounds where the only other atoms are metals or boron.
- Oxygen has an oxidation number of -2 in compounds, except for some compounds when combined with F, to which rules 2-4 apply. Oxygen, as the peroxide ion (O22-), has an oxidation number of -1.
- The elements of groups VA, VIA, and VIIA have oxidation numbers of -3, -2, and -1, respectively, when found in binary compounds with metals or hydrogen.
Two processes exist which can change the oxidation number of an atom, namely Oxidation and Reduction. When a substance is oxidized its oxidation number increases, and when a substance is reduced its oxidation number decreases; oxidation and reduction are reverse processes of each other.
The change in the oxidation number of an atom is the result of an exchange of electrons with another substance, either a loss or a gain of electrons. Oxidation involves the loss of electrons by a substance while reduction involves a substance gaining electrons. A common mnemonic device which is useful in remembering this is the phrase, “LEO the lion says GER”,
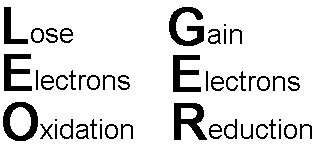
Lose Electrons
– Oxidation
Gain Electrons – Reduction
Reduction / Oxidation reactions always occur in pairs such that when one substance is oxidized another substance is reduced.
Oxidizing / Reducing agents
Certain substances are more likely than others to be either oxidized or reduced due to how likely they are to give away or gain electrons. The chemical property which relates how likely a substance is to gain an electron is called its “electronegativity”. Since a completed octet of electrons in the outermost shell of an atom is most stable, highly electronegative atoms tend to gain electrons and become reduced while less-electronegative atoms tend to lose electrons and become oxidized.
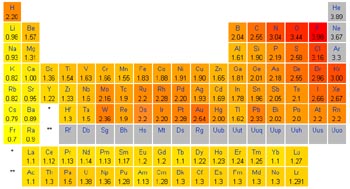
![]() Periodic Table
showing the electronegativity of each element using the
Pauling scale
Periodic Table
showing the electronegativity of each element using the
Pauling scale
Elements located on the left-most side of the periodic table have a high tendency to give up electrons to other atoms and be oxidized in order to achieve a completed octet in their outermost shell. These, and other, substances which have a tendency to give up electrons are often referred to as reducing agents since, as they are oxidized, they reduce other substances in the process. Reducing agents are oxidized during a redox reaction. Substances which are strong reducing agents include the Alkali and Alkaline-Earth elements (for example, Lithium, Sodium, Calcium, …) which are located in the first two columns of the periodic table.
On the other side of the periodic table, located just to the left of the Nobel gasses, are a group of elements which have a high tendency to gain electrons from other substances and be reduced. The Halogens, for example Fluorine and Chlorine are strong oxidizing agents, as are the Chalcogens with Oxygen being a prime example. During redox reactions, these substances become reduced as they oxidize other substances and are known as oxidizing agents. Oxidizing agents are reduced during a redox reaction.
Other, polyatomic, oxidizing and reducing agents exist in addition to pure elements. Common oxidizing substances include salts containing the Nitrate (NO3-), Chlorate (ClO3-), and Permanganate (MnO4-) ion; for example, KNO3, KClO3, and KMnO4. Ascorbic acid (also known as Vitamin C) as well as Hydrogen gas, Carbon Monoxide, and Hydrocarbons can act as a reducing agent in some reactions.
Half Reactions
Like any chemical reaction, a redox reaction must be balanced by mass, but additionally must also be balanced by charge so that the reaction obeys the laws of conservation of mass and charge. Because of this, Reduction and Oxidation reactions always occur in pairs; if one substance is oxidized, another substance must be reduced, and therefore charge is always conserved.
A
redox reaction can be broken up into two parts and analyzed
separately,
each called a half-reaction, one involving reduction and the
other
involving oxidation. Although individual half-reactions will
contain
free charge on either the reactant or product side, when a
pair of
balanced half-reactions are combined into a complete redox
reaction it
should contain no free charge since any electrons given up by
the
reducing agent will be gained by the oxidizing agent.
For example,
Consider the redox reaction which takes place between Zinc metal and Hydrochloric acid.
Compare the oxidation numbers / states of the reactants to the products. Initially, the Zinc is in its elemental form and is defined to have an oxidization number of zero. On the products side of the reaction the Zinc is part of an ionic compound [Zinc(II) Chloride] and now has an oxidization number of +2. During the reaction, the Zinc atom lost two electrons and was oxidized to become the Zn+2 ion. Now look at the Hydrogen on the reactants side; initially the Hydrogen’s oxidization number was +1. On the products side, however, the Hydrogen is in its elemental form and has an oxidization number of zero. During the reaction, the Hydrogen was reduced. Clearly a redox reaction is taking place. Chlorine’s oxidization number did not change during the reaction; it is merely a spectator ion and is not involving in the redox process.
With this new knowledge, we can write the ionic equation,
And we can infer the form of the two half-reactions to be,
In this case, the coefficients on the reactants / products were obtained from the coefficients from the full reaction, but this is not always the case since one might not know the full reaction to begin with, sometimes the coefficients must be altered to make sure both half-reactions are balanced by mass and charge. When the two half-reactions are combined, they should yield the full reaction and no longer contain any references to free electrons (they will cancel out since the same number of electrons will appear on both sides). In this reaction, the Zinc acts as the reducing agent, and is oxidized, and the Hydrogen ions act as the oxidizing agent, and are reduced.
Redox Reactions in Aqueous Solution
Other, arguably more complex, redox reactions may also occur when the reactants are in aqueous solution where the water itself takes part in the reaction. When construction / balancing the half reactions, it may be necessary to assume (due to the fact that the reactants are dissolved in water) that excess Hydrogen (H+) or Hydroxide (OH-) ions are present; the reaction will precede either under acidic or alkaline conditions. In this case, the water itself, or the ions it breaks into, becomes one of the reactants in the redox reaction even though it may not be entirely obvious initially.
For example,
Consider the reaction between the Permanganate ion (MnO4-) and the Ferrous, Iron +2, ion (Fe+2) in, acidic, aqueous solution.In this case, the cation of the Permanganate compound and the anion of the Fe+2 compound are, unimportant, spectator ions. The Permanganate ion is a strong oxidizer and will act as the oxidizing agent in this case while the Fe+2 ion will act as the reducing agent.
Since the Fe+2 ion is the reducing agent, it is oxidized in the process to become the Fe+3 ion.
As the Iron ion is oxidized, the Permanganate ion must be reduced. When the Permanganate ion is reduced it forms the Mn+2 ion and water. Now the fact that this reaction takes place in acidic conditions becomes important. The Oxygen from the Permanganate ion combine with the excess of H+ ions from acid solution to form water, leaving Mn+2 behind in slightly less-acidic solution.
These are the two half reactions which are balanced by mass. When these two reactions are combined in the right proportions such that the reaction is also balanced by charge we will have our complete redox reaction.
For every 1 oxidization reaction of an Fe+2 ion, 1 electron is released. It takes 5 electrons (in combination with 8 Hydrogen ions) to reduce the Permanganate ion to Mn+2 and 4 water molecules. Therefore, the Iron-oxidizing reaction must proceed at 5 times the rate as the Permanganate-reducing reaction. Taking this into account when combining the two half-reactions we find that the complete redox reaction is,
Unless we knew the reaction took place in acidic aqueous solution, it might be surprising to see that water is produced from this reaction even though no Hydrogen atoms are contained within the two most obvious reactants, namely the Fe+2 and Permanganate ions.
Redox Demonstrations
Aluminum’s capacity to act as a strong reducing agent is excellently demonstrated during a thermite reaction where it is used to reduce another, less reactive, metal oxide. The Aluminum is oxidized in the process, leaving the metal reduced to its elemental state and liberating a great deal of energy in the process.
Potassium Permanganate’s strong oxidizing nature is demonstrated in a reaction with glycerin. Shortly after the two substances are mixed, the Potassium Permanganate will automatically begin to rapidly oxidize / burn the glycerin in a very hot fire without even the need for external ignition.
|
Video
|
|---|
The
above
video illustrates a flame’s ability to reduce an oxidized
piece
of metal; these flames are known as 'reducing flames'. In the
above
video, a propane torch is used to heat a heavily-oxidized
piece of
Copper metal. Some of the propane fuel in the torch’s flame is
not
fully combusted due to insufficient Oxygen flow into the
torch’s
nozzle. The uncombusted fuel acts as the reducing agent for
the Copper
Oxide and one can see that, as the flame passes over a portion
of the
metal, bare (unoxidized) Copper becomes visible. When then
flame is
removed the hot Copper is again exposed to the Oxygen in the
air and
quickly oxidizes again, developing a black oxide layer.
Flames
which have excessive amounts of oxidizing gasses present will
act to
oxidize metal; such flames are known as 'oxidizing flames'.
Last updated: 03/13/2008